

Stay ahead of ever-changing global regulations with a smart compliance engine that ensures continuous audit-readiness, no matter the market or molecule.
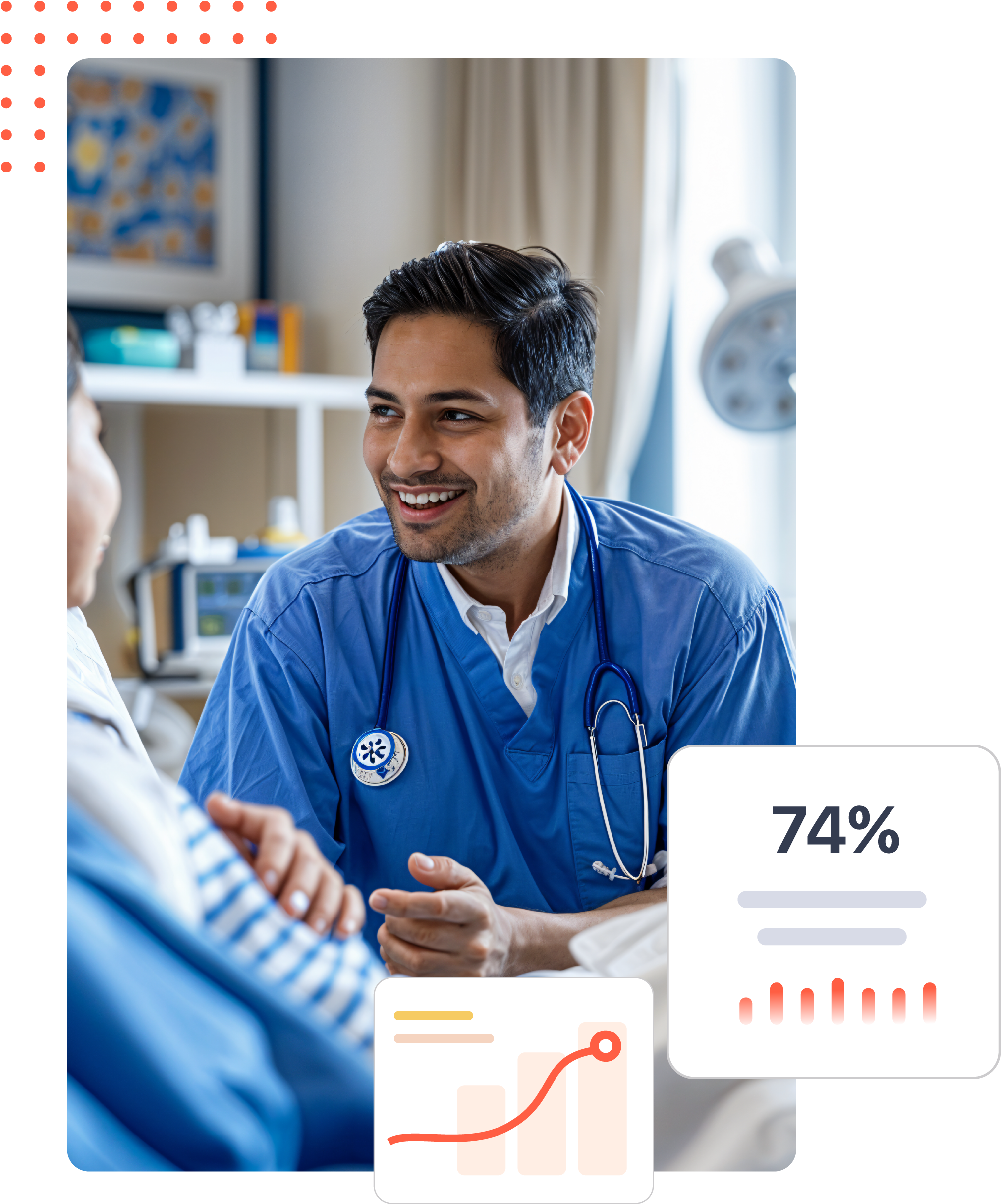
Deliver laboratory accuracy at scale with automated workflows, real-time validations, and digital traceability for every test and trial.
Globally compliant, locally confident

Validated results. Uncompromised safety

Where quality meets vigilance
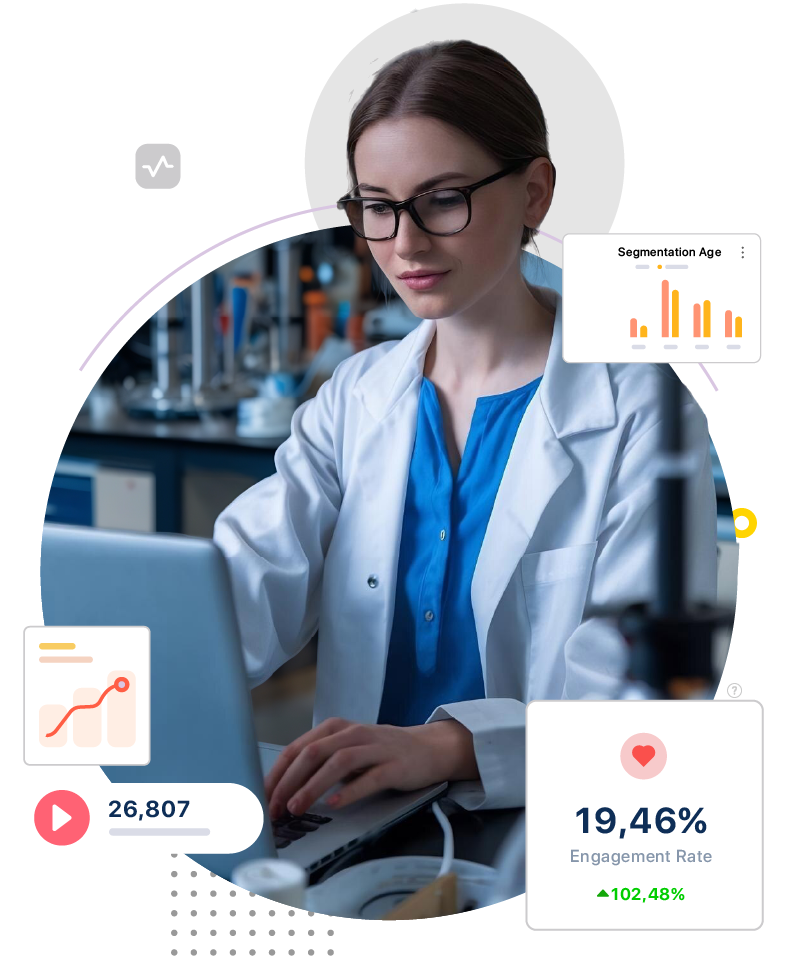
Usage : Ensures adherence to global standards like GMP, GLP, GCP, ISO 13485, FDA, MHRA, and EMA with automated documentation, compliance workflows, and audit readiness tools.
Impact : Eliminates non-compliance risks, shortens approval timelines, and improves cross-border readiness for pharmaceutical and med-device manufacturers.
Usage : Digitally manage microbiological, chemical, and clinical laboratory testing with traceable results and automated validation checks.
Impact : Speeds up lab throughput, reduces human error, and ensures consistency in quality for drugs, supplements, and medical devices.
Certify fast. Compete faster
Where science meets certainty
Label smart. Pack right. Go global
Predict. Prevent. Protect

Usage : Automates quality inspections, failure mode tracking, and batch-level product safety reporting across production lines.
Impact : Enhances end-to-end product quality and reduces safety-related recalls or investigations.

Usage : Real-time digital certification platform to manage ISO, CE marking, FDA approvals, and custom declarations.
Impact : Reduces paperwork, accelerates global go-to-market readiness, and instills trust across stakeholders.
Usage : Manage clinical testing—from sample collection to stability testing, bioanalysis, and electronic data submissions—with full compliance and accuracy.
Impact : Accelerates clinical trial cycles, enhances participant safety, and ensures data defensibility.
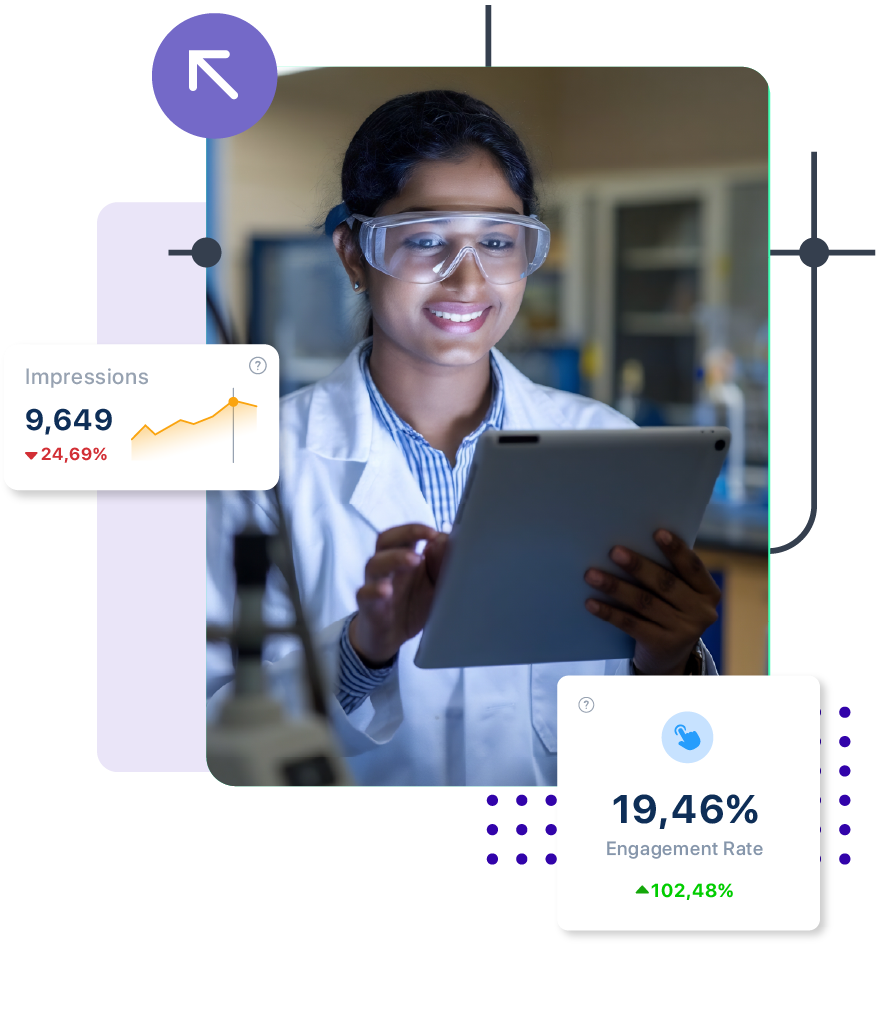

Usage : Automates validation of packaging standards, labeling (QR, RFID), and artwork compliance based on country-specific regulations.
Impact : Prevents regulatory fines, reduces market entry errors, and enables sustainable, compliant packaging.
Usage : Integrates preclinical risk assessment tools, toxicology profiles, and exposure modeling across cosmetics, pharma, and nutraceuticals.
Impact : Accelerates safety assessments, minimizes animal testing, and improves formulation safety.

Usage : Supports on-site and remote inspections with guided checklists, video collaboration, document traceability, and automated CAPA tracking.
Impact : Reduces audit preparation time, ensures continuous readiness, and enhances inspector confidence.
Usage : Covers design validation, performance, EMC, electrical safety, biocompatibility, and usability testing across the device lifecycle.
Impact : Ensures regulatory compliance, product durability, and safe patient outcomes.
Usage : Tracks and certifies raw materials, APIs, and excipients with provenance, contamination checks, and vendor scorecards.
Impact : Builds a resilient and transparent supply chain to minimize recalls and build consumer trust.
Usage : Enables environmental testing for pharmaceutical effluents, biodegradability, and workplace exposure—aligned with EPA and REACH regulations.
Impact : Mitigates ecological risks, supports ESG goals, and ensures sustainability across labs and plants.
Create tamper-proof product journeys—from raw material to retail—with integrated blockchain traceability and digital vendor assurance
Deliver confidence to every consumer with AI-reviewed claims, dermatological safety testing, and transparent product validation
Monitor, measure, and minimize your environmental footprint with science-backed testing, GHG tracking, and ESG-ready insights